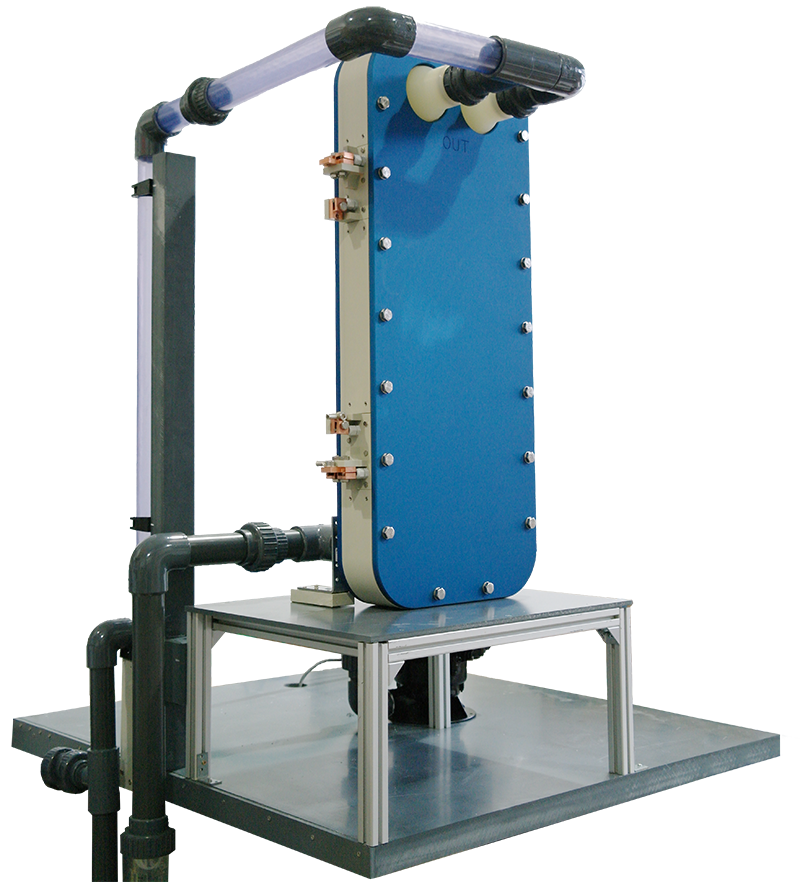
The state of scientific research
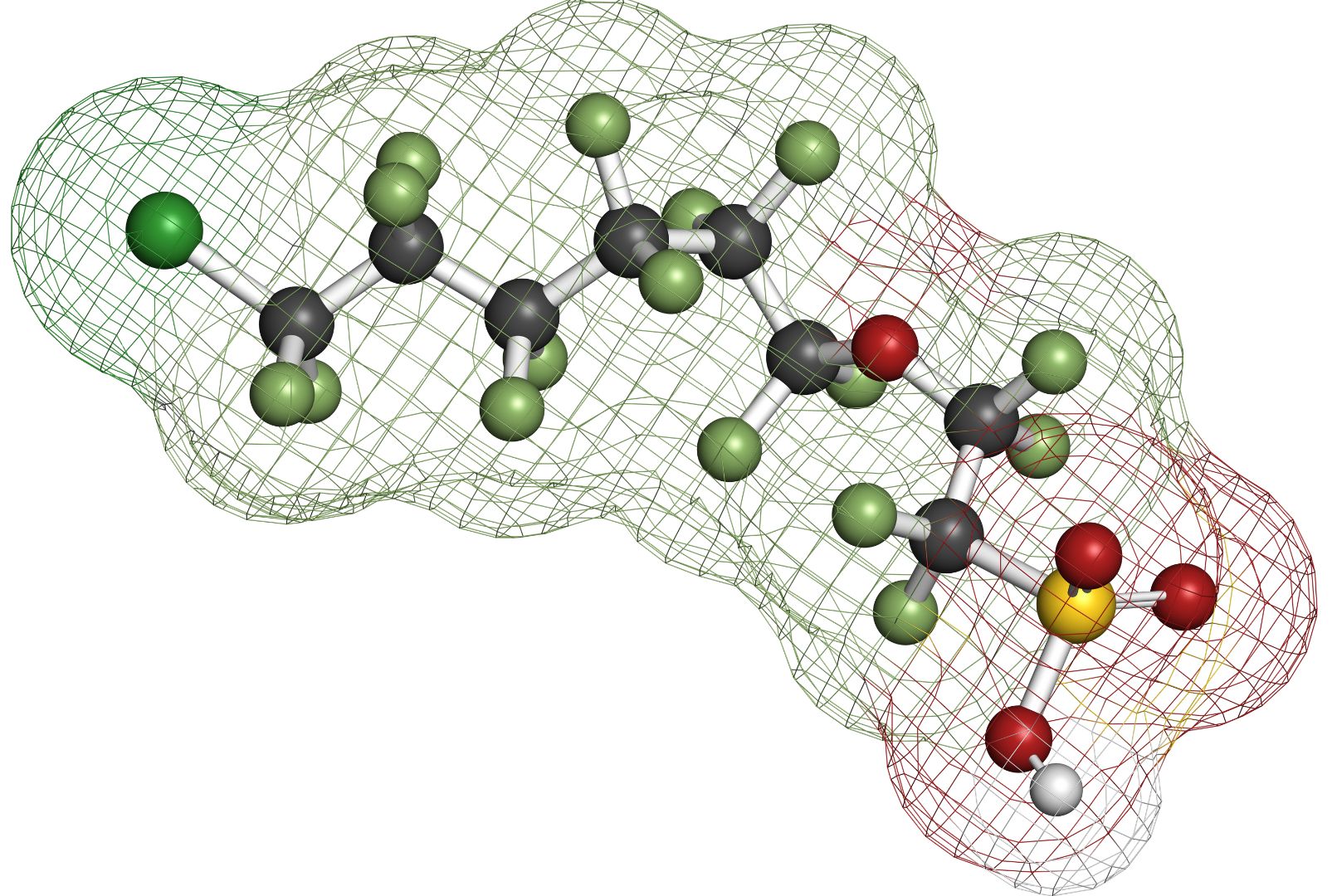
Research into the use of boron-doped diamond (BDD) electrodes for the remediation of PFAS-contaminated soils and waters through anodic oxidation has yielded highly promising results. This method has demonstrated that many widely occurring PFAS compounds can be effectively degraded and ultimately mineralized into the inorganic end products CO₂, fluoride ions, and, depending on the PFAS structure, sulfate. Particularly noteworthy is the efficiency of this technology in treating short-chain PFAS, which are considered especially difficult to degrade due to their chemical stability and environmental persistence.
A crucial factor for the success of the treatment is the anode potential, which can be influenced by the current density and significantly determines the course of the reactions. Similarly, the choice of electrolyte and the pH value of the solution play a significant role in the effectiveness of the process.
The high effectiveness of BDD electrodes in the electrooxidation of PFAS is due to their large overpotential for oxygen, which allows minimizing undesirable side reactions. This makes BDD electrodes exceptionally well-suited for PFAS remediation and offers promising applications in practice. Additionally, this method is characterized by its sustainability and the potential for large-scale application, making it a promising solution for addressing PFAS contamination in the environment.
Highlights
- We have compiled a selection of interesting scientific studies on the degradation of various PFAS using BDD electrolysis for you – you can find them below in the list of sources.
- Currently, BDD electrolysis cells from pro aqua are being used in several projects worldwide for PFAS degradation. We will provide updates on these projects in future blog posts.
Optimal application areas of BDD electrolysis cells in PFAS remediation
Treatment of highly contaminated waters
BDD electrodes are particularly useful for the treatment of waters heavily contaminated with PFAS, as they can efficiently break down high PFAS concentrations. This is used, for example, for the treatment of groundwater contaminated by PFAS-containing firefighting foams.
Treatment of pre-concentrated wastewater
BDD electrodes are ideally suited for the treatment of PFAS-containing wastewater, where PFAS concentrations have been significantly increased through pre-treatment methods such as reverse osmosis.
Regeneration of PFAS adsorption materials
BDD electrodes can also be used to regenerate adsorption materials such as activated carbon or ion exchange resins that are used for the treatment of PFAS-contaminated wastewater before discharge into the sewage system, industrial process water, or drinking water. During regeneration, the PFAS accumulated in the adsorption materials are rinsed out and subsequently electrochemically degraded in the regenerant solution.
List of sources
Are you looking for research results related to the degradation of specific PFAS compounds?
In unserem Quellenverzeichnis finden Sie einen Überblick. Bei Fragen helfen Ihnen unsere Expert:innen gern weiter.
- Charles E. Schaefer, Sarah Choyke, P. Lee Ferguson, Christina Andaya, Aniela Burant, Andrew Maizel, Timothy J. Strathmann, and Christopher P. Higgins, "Electrochemical Transformations of Perfluoroalkyl Acid (PFAA) Precursors and PFAAs in Groundwater Impacted with Aqueous Film Forming Foams," Environmental Science & Technology, 2018, Vol. 52(18), pp. 10689–10697. https://doi.org/10.1021/acs.est.8b02726
- Beatriz Gomez-Ruiz, Nazely Diban, Ane Urtiaga, "Comparison of microcrystalline and ultrananocrystalline boron doped diamond anodes: Influence on perfluorooctanoic acid electrolysis," Separation and Purification Technology, 2018, Vol. 208, pp. 169–177. https://doi.org/10.1016/j.seppur.2018.03.044
- Diwakar Suresh Babu, Johannes M.C. Mol, Josephus G. Buijnsters, „Experimental insights into anodic oxidation of hexafluoropropylene oxide dimer acid (GenX) on boron-doped diamond anodes,“ Chemosphere, 2022, Vol. 288(1), Article 132417.
https://doi.org/10.1016/j.chemosphere.2021.132417 - Hanshuang Xiao, Baoying Lv, Guohua Zhao, Yujing Wang, Mingfang Li, and Dongming Li, „Hydrothermally Enhanced Electrochemical Oxidation of High Concentration Refractory Perfluorooctanoic Acid,“ The Journal of Physical Chemistry A, 2011, Vol. 115(47), pp. 13836–13841.
https://doi.org/10.1021/jp207519j - A. M. Trautmann, H. Schell, K. R. Schmidt, K.-M. Mangold, A. Tiehm, „Electrochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in groundwater,“ Water Science and Technology, 2015, Vol. 71(10), pp. 1569–1575.
https://doi.org/10.2166/wst.2015.143 - Tsuyoshi Ochiai, Yuichi Iizuka, Kazuya Nakata, Taketoshi Murakami, Donald A. Tryk, Akira Fujishima, Yoshihiro Koide, Yuko Morito, „Efficient electrochemical decomposition of perfluorocarboxylic acids by the use of a boron-doped diamond electrode,“ Diamond and Related Materials, 2011, Vol. 20(2), pp. 64–67.
https://doi.org/10.1016/j.diamond.2010.12.008 - Qiongfang Zhuo, Shubo Deng, Bo Yang, Jun Huang, Bin Wang, Tingting Zhang, Gang Yu, „Degradation of perfluorinated compounds on a boron-doped diamond electrode,“ Electrochimica Acta, 2012, Vol. 77, pp. 17–22.
https://doi.org/10.1016/j.electacta.2012.04.145 - Ane Urtiaga, Alvaro Soriano, Jordi Carrillo-Abad, „BDD anodic treatment of 6:2 fluorotelomer sulfonate (6:2 FTSA). Evaluation of operating variables and by-product formation,“ Chemosphere, 2018, Vol. 201, pp. 571–577.
https://doi.org/10.1016/j.chemosphere.2018.03.027 - Ane Urtiaga, Carolina Fernández-González, Sonia Gómez-Lavín, Inmaculada Ortiz, „Kinetics of the electrochemical mineralization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes,“ Chemosphere, 2015, Vol. 129, pp. 20–26.
https://doi.org/10.1016/j.chemosphere.2014.05.090 - Hui Lin, Junfeng Niu, Jiale Xu, Haiou Huang, Duo Li, Zhihan Yue, and Chenghong Feng, „Highly Efficient and Mild Electrochemical Mineralization of Long-Chain Perfluorocarboxylic Acids (C9–C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD Electrodes,“ Environmental Science & Technology, 2013, Vol. 47(22), pp. 13039–13046.
https://doi.org/10.1021/es4034414