From electrolysis to diamond electrodes
To explain how the diamond electrode works and the effects it has, one needs to step into the field of electrochemistry. Based on the chemical process of electrolysis, it can be demonstrated step by step how the diamond electrode can be used sustainably in the field of water treatment and disinfection.
Electrolysis
in simple terms
Put simply, electrolysis is nothing more than separating a chemical compound using electricity. In other words, electrical energy is converted into chemical energy, which is why electrolysis is referred to as an electrochemical process.
In the electrolysis of water, for example, it means that water (H2O), is separated into the individual chemical elements hydrogen H2 and oxygen O2 with the help of electricity. But more on that later.
Electrolysis is therefore the counterpart or reverse process of a battery or accumulator when discharging, as a galvanic cell converts chemical energy into electrical energy. Electrolysis is then again required during the charging process.
Electrolysis is the conversion of electrical energy into chemical energy
How does electrolysis work?
Electrolysis requires an electrically conductive solution (electrolyte) in which two electrodes are immersed as well as a voltage source.
If the electrodes immersed in the electrolyte are connected to the voltage source, a shortage of electrons at the electrode (= anode) connected to the positive pole occurs. The electrode (= cathode) that is connected to the negative pole, on the other hand, experiences an excess of electrons.
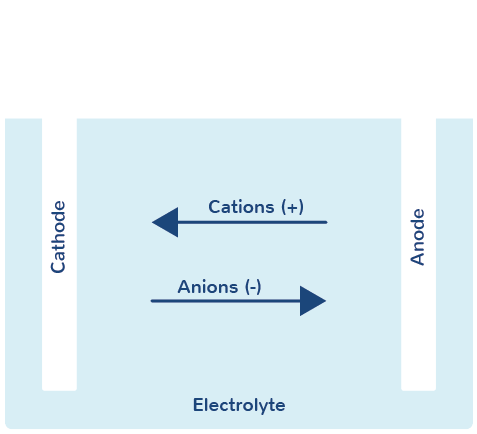
Negatively and positively charged ions
The electrolyte contains dissolved positive and negative ions.
The negatively charged ions (= anions) migrate to the anode and lose their electrons to the electrode (= oxidation).
The positively charged ions (= cations) conversely migrate to the cathode and acquire electrons (= reduction).
What is a redox reaction?
A chemical reaction in which an electron transfer takes place is called a redox reaction. In electrolysis, the transfer of electrons takes place on the surfaces of the electrodes and consists of two partial reactions: Electrons are lost on one side and acquired on the other.
The loss of electrons is called oxidation, the acquisition of electrons is called reduction.
Which redox reactions then take place ultimately depends on the nature of the electrolyte and the material of the electrodes.
Did you know?
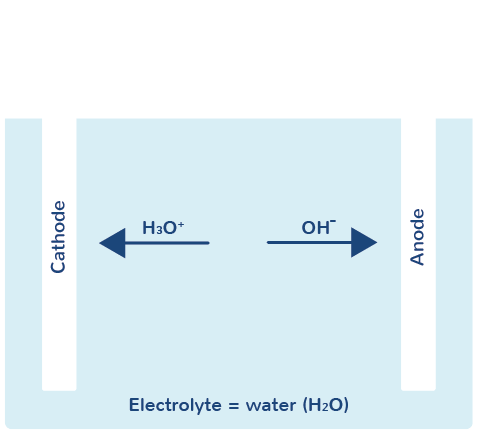
What happens during the electrolysis of water?
The electrolysis of water is the decomposition of the electrolyte water H2O under the influence of electricity into oxygen O2 and hydrogen H2. Water undergoes what is known as autoprotolysis and thus exhibits electrical conductivity. This means that water is not only present in its pure form as H2O, but that oxonium ions (H3O+) and hydroxide ions (OH-) are formed.
If voltage is now applied to the electrodes, the negatively charged hydroxide ions (= anions) migrate to the anode and oxygen O2 is formed. The positively charged oxonium ions (= cations) migrate to the cathode and hydrogen H2 is formed.
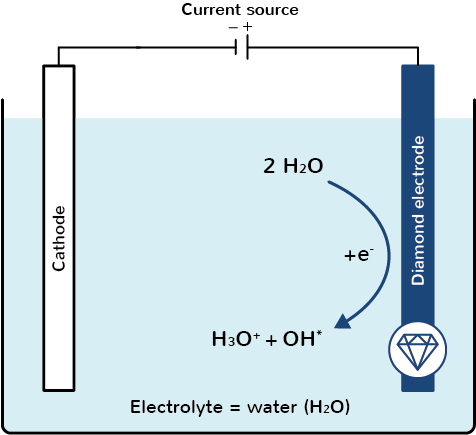
Why water electrolysis with boron-doped diamond electrodes?
However, if water electrolysis is carried out with boron-doped diamond electrodes, then primarily OH radicals (OH*) are formed on the surface of the anodic diamond electrode (source: Michaud, 2002).
OH radicals are among the strongest known oxidants and cannot be generated by any other electrodes available on the market, or not to this extent (source: Brillas, 2016).
The special thing about oxidants is that they can acquire electrons and reduce themselves in the process.
Did you know?
OH radicals are also produced in the atmosphere by an interaction between ozone, water and UV rays. They are known as the detergents of the atmosphere, as they contribute significantly to the decomposition of air pollutants.
More Power:
Overvoltage makes it possible!
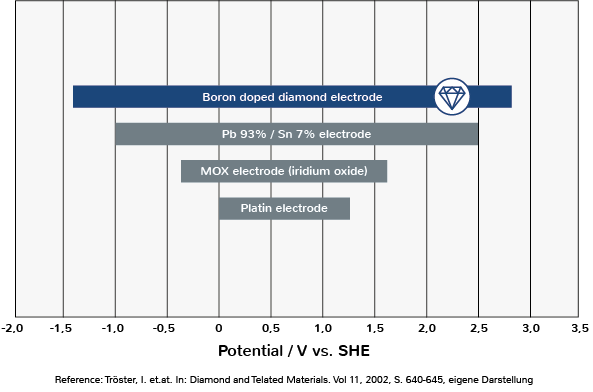
According to the theory, water electrolysis takes place when the necessary decomposition voltage is applied. This corresponds to the voltage that results from the difference between the electrode potentials of the anode and cathode. However, practice shows that the decomposition voltage is not sufficient to trigger water electrolysis, but must be increased by the so-called overvoltage. The overvoltage is thus the difference between the actually measured voltage (terminal voltage) and the calculated decomposition voltage (Nernst equation).
The overvoltage is determined, among other things, by the electrode material, the nature of the electrode surface, the electrolyte, the current and the temperature. The boron-doped diamond electrode with its high overvoltage (source: Tröster, 2002; Song, 2018) is capable of generating large quantities of OH radicals in water electrolysis (source: N. Rabaaoui, 2012).
Did you know?
The boron-doped diamond electrode is inert and therefore does not dissolve during the electrochemical process. Tin or lead electrodes would also produce high overvoltages, but these dissolve, contaminating the electrolyte.
INNOVATION AND QUALITY:
The pro aqua diamond electrode
The pro aqua diamond electrode is pro aqua’s core technology and the base for all its products. The electrode's carrier material is a fluorinated and thus chemically resistant plastic, which is equipped with boron-doped diamond particles. Boron doping ensures the electrical conductivity of the diamond particles, which generally are not electrically conductive at all. The electrical conductivity is responsible for the fact that the special electrochemical properties of the diamonds can only be used in electrolysis.
The standard area of a pro aqua diamond electrode is 700cm2 (260x270x0.5 mm). Electrodes beyond the standard size are currently limited to a width of 540mm. Yet they could theoretically be produced in any length. By cutting, smaller electrodes can be obtained from the standard electrode.
pro aqua's diamond electrodes are long-lasting and stable.
Operating times of more than 20,000 hours can be achieved.
Simple installation, quick commissioning
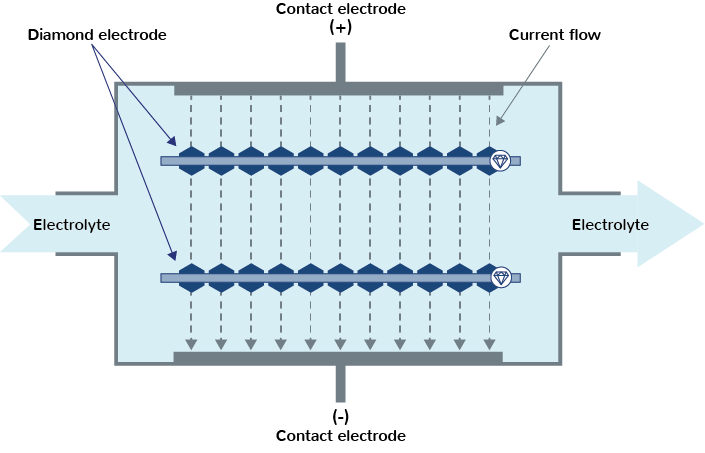
The pro aqua diamond electrode is flexible (can be bent) = elastic because the carrier material is fluorinated plastic. The electrode must also be operated in a bi-polar manner. These technical properties require the diamond electrodes to be carefully and expertly installed in a flow cell (= electrochemical cell). The flow cells have an electrolyte inlet and outlet, two electrical connections for the power supply and corresponding devices for assembly. The assembly and commissioning of the flow cell is therefore simple and quick.
The correct handling of diamond particles involves:
The bi-polar mode of operation
The carrier material (plastic) of the pro aqua diamond electrode is electrically non-conductive, which means the diamond particles must be supplied with power via a bi-polar operating mode. For this purpose, one or more diamond electrodes are installed between two contacting electrodes in a flow cell.
The contacting electrodes are supplied with voltage and the electrolyte to be treated, e.g. water, is pumped through the cell. The electrical conductivity of the electrolyte and the special arrangement of the boron-doped diamond particles in the plastic matrix cause the current to flow from the contacting electrode (anode) to the contacting electrode (cathode) via the diamonds.
Water treatment using the diamond electrode:
One technology, two processes
The current activates the boron-doped diamonds, which then generate oxidants such as OH radicals directly from the electrolyte. This is then referred to as electrochemically-activated water. The oxidants present in the electrochemically-activated water react with the pollutants (viruses, bacteria, organic matter, etc.) present in the (waste) water and break them down.
The flow cells can be operated according to the in-situ or ex-situ principle.
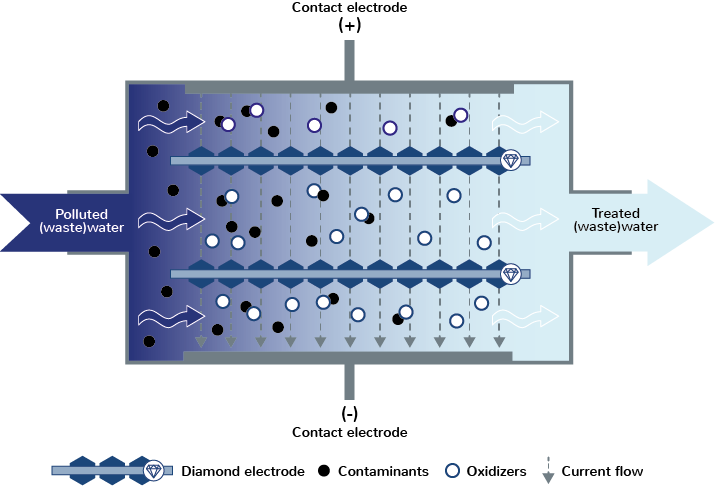
1. In-situ: Pollutant degradation directly in the cell
The in-situ principle is based on a contaminated electrolyte, e.g. waste water, being pumped through the flow cell. The oxidants produced by the electrolysis act on the pollutants such as bacteria or organic matter directly in the flow cell and eliminate/degrade them.
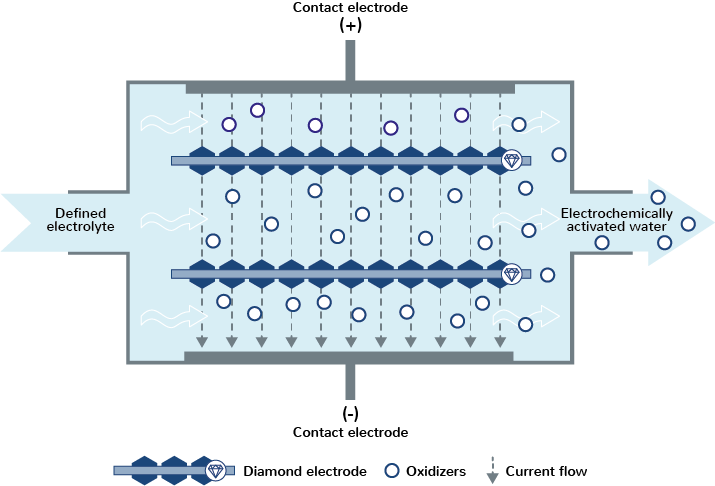
2. Ex-Situ: Pollutant degradation by means of electrochemically-activated water
The ex-situ principle is based on pumping a defined electrolyte, e.g. water with a defined salt content, through the flow cell. After electrolysis, water enriched with an oxidant (= electrochemically-activated water) is available, which is used, among other things, for cleaning and disinfecting surfaces and equipment, washing and disinfecting fruit, vegetables, etc. and for plant and animal protection.
You are interested in our products or cooperation?

